Filing Customs Declarations Using the Customs Declarations UK Platform
The Customs Declarations UK (CDUK) platform provides UK importers with a streamlined, validated pathway for submitting import declarations to HMRC’s Customs Declaration Service. For pharmaceutical importers managing complex, high-value, and highly regulated products, CDUK offers critical advantages: guided workflows that capture all mandatory data elements, real-time validation to identify errors or omissions before submission, and secure archiving of declaration records for the statutory six-year retention period.
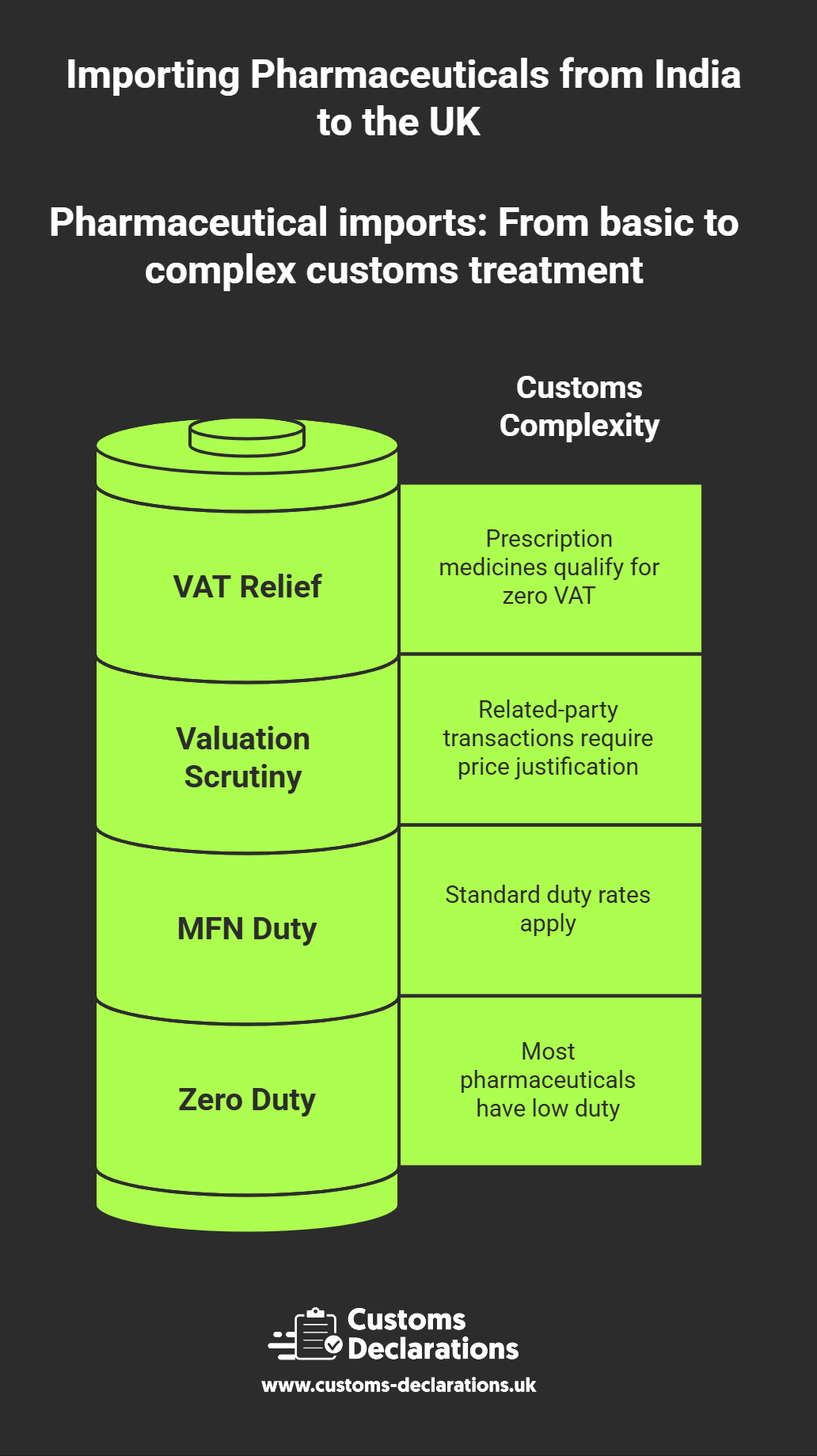
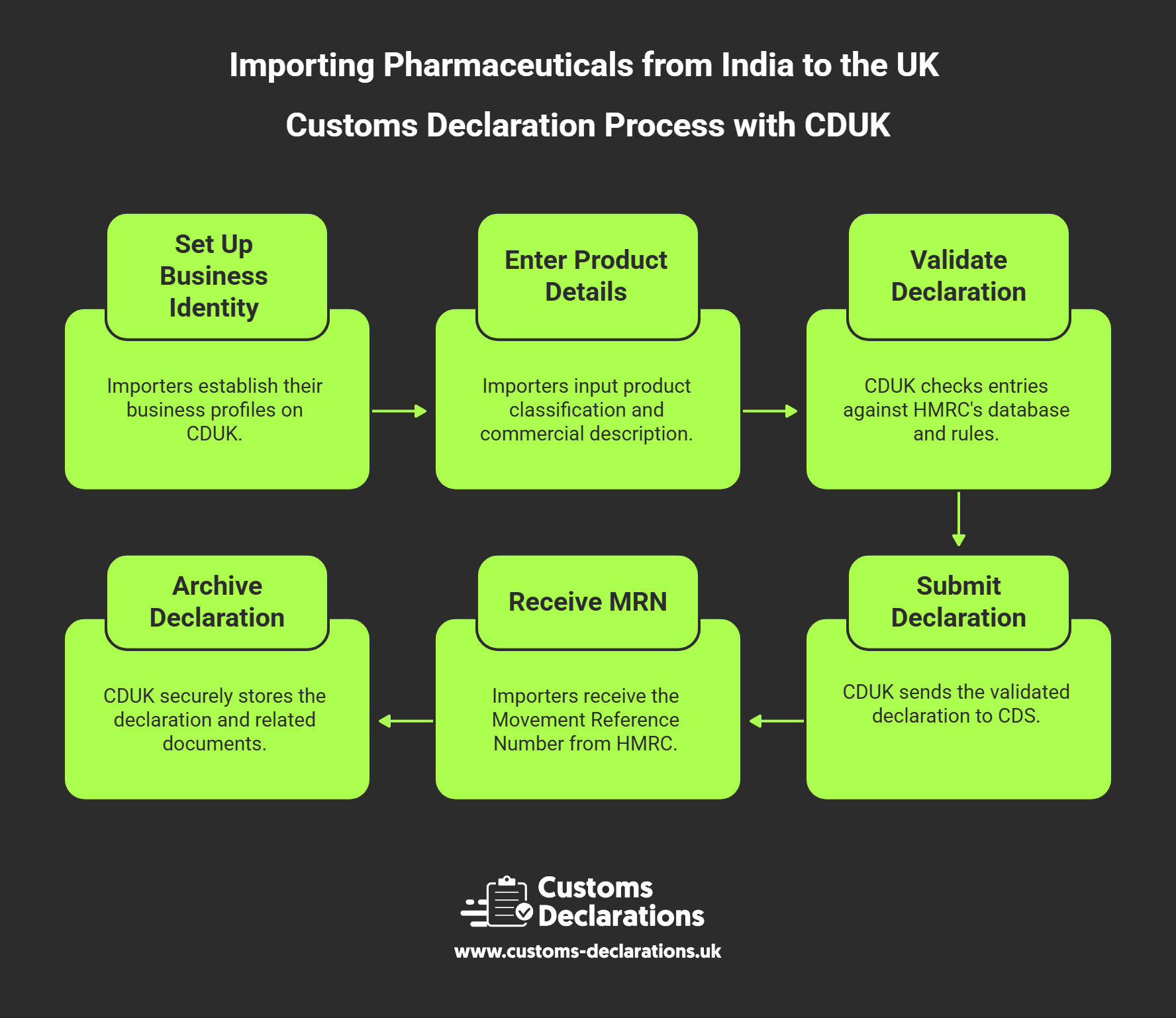
Within CDUK, importers set up their business identities—importer EORI, declarant details, and any customs broker acting on their behalf—once, and then reuse these profiles across multiple declarations. For each pharmaceutical import, the platform guides the user through entering the product classification (the ten-digit commodity code), the commercial description (which should match the regulatory approval and invoice), the customs value and its components (FOB price, freight, insurance), the country of origin, and any applicable preference or relief claims. CDUK’s validation engine cross-checks these entries against HMRC’s tariff database and business rules, flagging issues such as missing licence references, incorrect VAT indicators, or mismatched values before the declaration is transmitted.
Once the declaration is complete and validated, CDUK submits it electronically to CDS and returns the Movement Reference Number (MRN) upon HMRC acceptance. The MRN serves as the official clearance reference and must be provided to the freight forwarder or port operator to release the goods. CDUK retains the full declaration dataset, supporting documents, and status updates in a secure, searchable archive, enabling importers to respond instantly to HMRC post-clearance queries, MHRA inspections, or internal audit reviews. For pharmaceutical businesses managing dozens or hundreds of product lines, CDUK’s template and cloning features allow rapid replication of declaration structures, reducing manual entry and ensuring consistency across shipments.
Additionally, CDUK facilitates the submission of Entry Summary Declarations (ENS) for safety and security compliance, ensuring that advance cargo information aligns with the customs declaration and preventing border delays. By integrating both CDS declarations and ENS declarations in a single platform, pharmaceutical importers gain end-to-end visibility and control over the clearance process, from pre-arrival notification through final release.