Introduction
Cosmetics exports—from skincare and colour cosmetics to fragrances and aerosols—offer compelling growth for UK brands. Yet the path to a new market runs through a dense mesh of product-safety rules, market-specific registrations, exacting labelling norms, and precise border formalities. Treat these requirements as a single, end-to-end process—beginning with formula review and ending with a validated customs declaration—and export becomes repeatable, auditable, and timely. The overview below distils the essentials for compliant market entry while showing where the Customs Declarations UK (CDUK) platform streamlines the declaration step.
First Principles: What a “Cosmetic” Is—and Why It Matters
The legal category drives everything that follows. In the UK/EU framework, “cosmetic” covers products applied to external parts of the body to clean, perfume, change appearance, protect, or keep them in good condition, whereas the same cream in the United States may be a cosmetic, a drug, or both—depending on the claims you make. Labelling a moisturiser as “treats acne” or “stimulates hair growth” moves it into drug territory and triggers different pre-market obligations. Getting this definition right prevents refusals, detentions, and recalls.
Governance and Roles: Responsible Persons and Market Notifications
In the European Union, a local Responsible Person (RP) must be appointed. The RP is legally accountable, ensures product notification via the CPNP portal, and maintains the technical file. Great Britain mirrors many elements: appoint a UK Responsible Person and notify products on the SCPN. These obligations sit alongside country-specific rules in priority destinations like the United States (MoCRA facility registration and product listing), Canada (Cosmetic Notification Form within 10 days of first sale), and China (CSAR registration/notification through a domestic representative). Early mapping of these roles and portals prevents last-minute blocks at release.
Safety & Quality System: Build the Technical Backbone
Across major markets the fundamentals converge: maintain ISO 22716 (GMP); compile and keep current a Product Information File with a Cosmetic Product Safety Report (CPSR); substantiate performance claims; and ensure every ingredient is permitted in the destination market. The EU has long banned and restricted many substances; China applies monitoring for new cosmetic ingredients; and the U.S. pays special attention to colour additives and truthful claims. Audit your formula, suppliers, and evidence annually and whenever the product changes.
Labelling Without Errors: Universal Elements and Local Nuances
A label is your passport at the border: if it is incomplete or in the wrong language, customs can refuse entry. As a baseline, include the product identity, net contents, INCI ingredient list in descending order, name and address of the RP or local distributor, country of origin, required warnings, batch/lot code, and durability (expiry date or PAO symbol). Many markets require local language(s)—for example, bilingual English/French in Canada and Arabic alongside English across the Gulf—so plan artwork and over-labels early. Recent EU updates expanded the list of declarable fragrance allergens; factor transition periods into your print schedule.
Regulators also police claims. In the EU/UK, the six common criteria in Regulation (EU) 655/2013 require claims to be legal, truthful, evidence-based, and fair; in the U.S., drug-like claims demand compliance with OTC or NDA pathways. Build claim substantiation into product development to avoid rework.
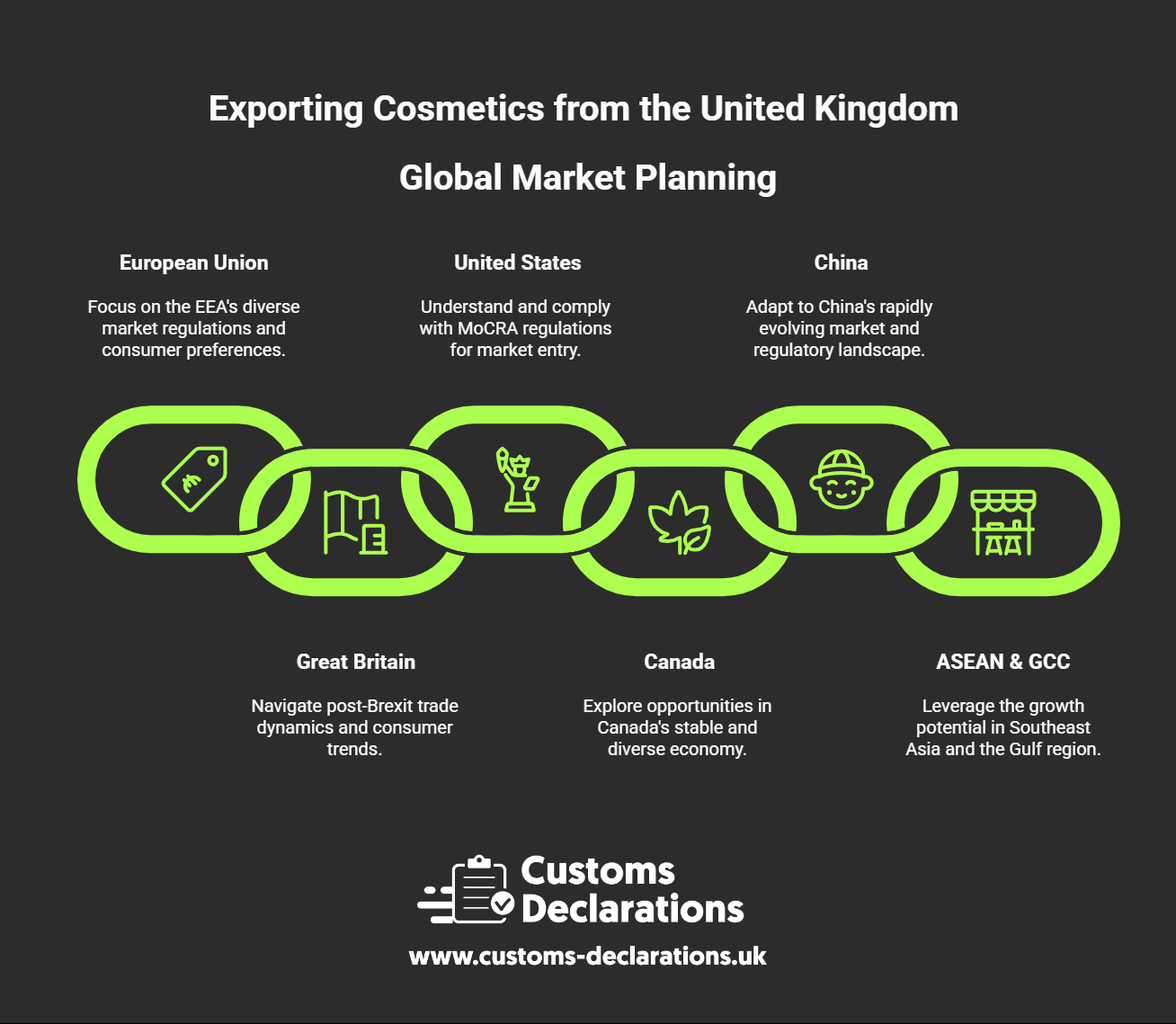
Market-by-Market Highlights You Should Plan For
European Union (EEA). Appoint an EU RP, notify on CPNP, maintain PIF/CPSR and GMP, and ensure claims meet the common criteria.
Great Britain. Appoint a UK RP and notify on SCPN; GB policy mirrors many EU elements while operating under its own portals and marking.
United States (MoCRA). Register facilities, list products, maintain safety substantiation, and report serious adverse events in defined windows; labels must meet 21 CFR Part 701.
Canada. File the CNF within 10 days of first sale and follow Canadian labelling (INCI and bilingual text).
China. Under CSAR, “general” cosmetics are notified, “special” categories are registered; animal-testing exemptions exist subject to conditions, and local representation is required.
ASEAN & GCC. The ASEAN Cosmetic Directive harmonises core requirements but uses national notifications; in Saudi Arabia and the UAE, expect eCosma/GHAD or ECAS/Montaji registration checks at customs and Arabic/English labels.
Dangerous Goods and Transport Integrity
Fragrances and many aerosols are flammable due to alcohol or propellants. Airlines and shipping lines apply IATA/ICAO and IMDG/ADR rules; shipments typically require SDS documentation and, where available, limited-quantities provisions. Align packaging, carrier selection, and documentation to avoid rejections at tender or uplift.
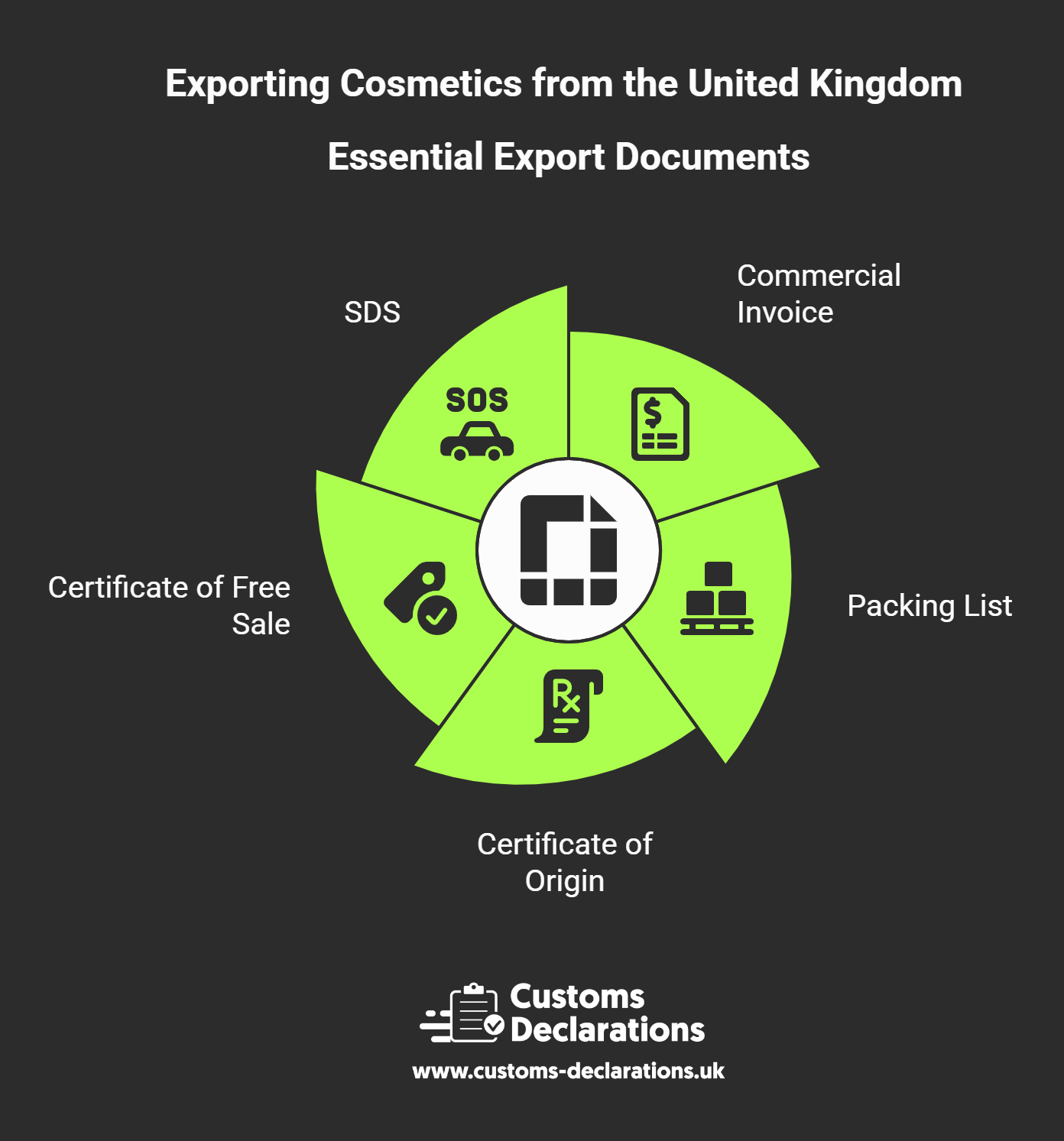
Build the Export Dossier: Documents That Travel Well
Your file should present a coherent, verifiable picture of the goods:
- Commercial invoice with clear description, value, origin, and agreed Incoterm;
- Packing list that mirrors the invoice and identifies weights, dimensions, and carton contents;
- Certificate of Origin where preferential tariffs are claimed;
- Certificate of Free Sale if required by the destination; and
- SDS for any dangerous goods.
These items are the foundation of both border clearance and downstream post-clearance audits—keep complete digital copies aligned to each shipment.
Electronic Declarations: Filing Through Customs Declarations UK (CDUK)
Every export from Great Britain requires a Full export declaration in HMRC’s Customs Declaration Service (CDS), supported by your GB EORI. Within CDUK, the workflow is structured in plain English: select the export procedure for cosmetics, enter line-item values and quantities, attach licence or certificate references, indicate preference where relevant, and submit for validation. CDUK runs real-time checks to catch inconsistencies before transmission. When HMRC accepts the entry, a Movement Reference Number (MRN) appears in the notification area for sharing with the forwarder and for long-term record-keeping.
Deep-sea containers typically require the declaration to be lodged well ahead of loading; road, short-sea, and air have stricter cut-offs. Using a platform that surfaces these timing rules reduces roll-overs and storage charges.
For step-by-step guidance, see CDUK’s knowledge base on export declarations and cds declarations (internal links).
Safety & Security Data (ENS) and Port Readiness
Carriers are responsible for safety and security filings on outbound legs, but they depend on your accurate descriptions and weights. Discrepancies between those filings and the customs declaration can trigger holds or secondary screening, particularly at trans-shipment ports. Align product narratives and unit measures across commercial documents, declarations, and carrier data. For background on the data set and timing, review CDUK’s primer on ens declarations.
Pricing, Terms, and Insurance: Align the Contract to the Compliance
Choice of Incoterms® determines who files and who pays. Under EXW, the buyer handles UK export formalities and risk much earlier in the journey; under CIP, you control carriage and insurance to the named place, which may be preferable for temperature-sensitive or high-value consignments. Match your marine-cargo insurance limits and deductibles to retailer or lender covenants for cosmetic launches with significant advertising spend.
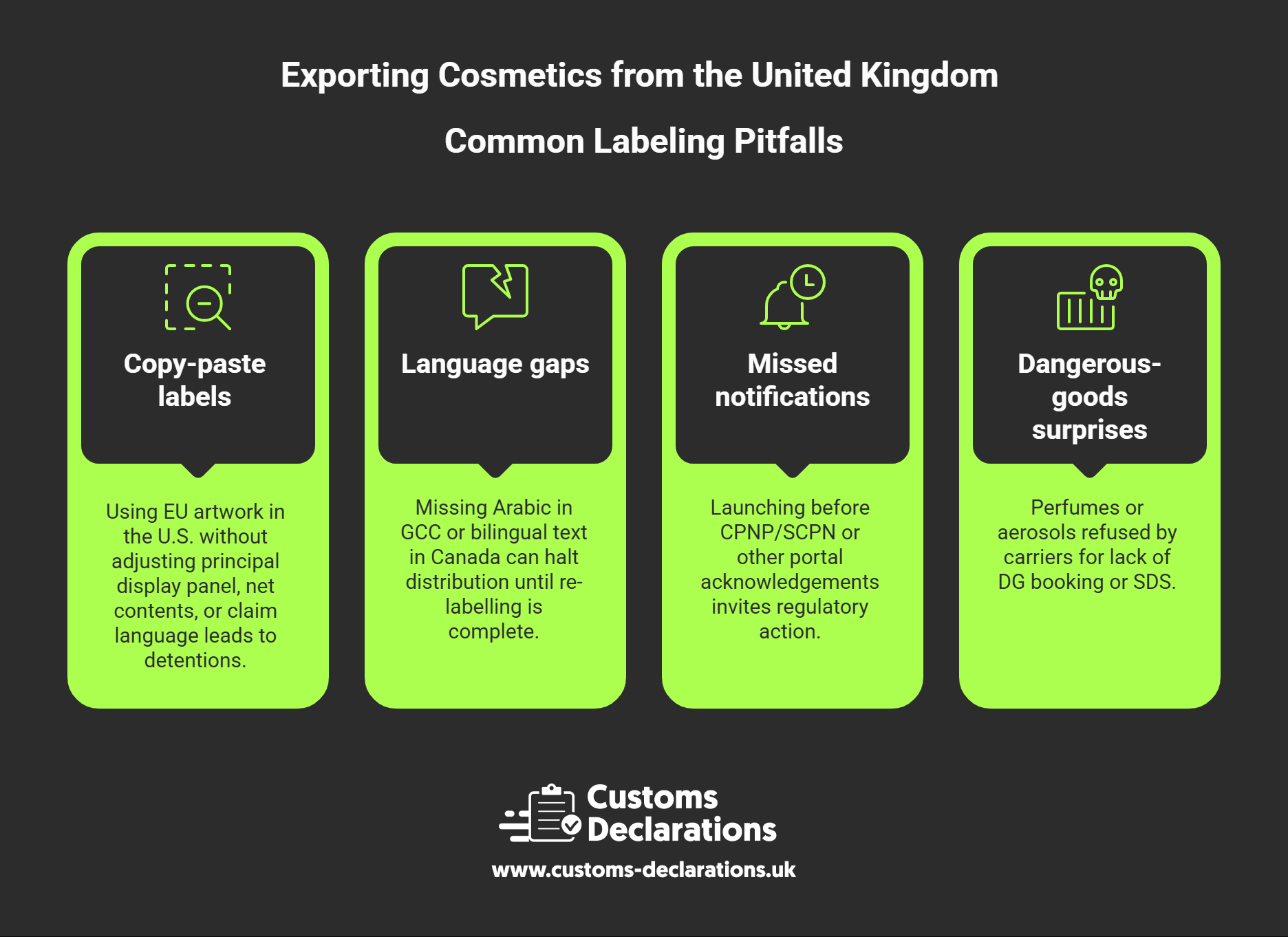
Common Pitfalls—and How to Prevent Them
Copy-paste labels. Using EU artwork in the U.S. without adjusting principal display panel, net contents, or claim language leads to detentions.
Language gaps. Missing Arabic in GCC or bilingual text in Canada can halt distribution until re-labelling is complete.
Missed notifications. Launching before CPNP/SCPN or other portal acknowledgements invites regulatory action.
Dangerous-goods surprises. Perfumes or aerosols refused by carriers for lack of DG booking or SDS.
Turn these into controls: lock a pre-shipment checklist into your NPI process; use CDUK templates for repeat SKUs; and keep declaration data synchronised with approved label copy and the technical file.
Conclusion
Exporting cosmetics at scale is entirely manageable when safety documentation, labelling, and market notifications are treated as part of product development—not an afterthought. The remaining variables—carrier rules, security filings, and the customs declaration—are best addressed through disciplined document control and a robust, validated filing flow. By integrating these strands and using the Customs Declarations UK platform for your CDS submissions, UK exporters deliver consignments on schedule, in full compliance, and with an audit trail ready for any regulator or retailer.